58+ Atom Nucleus Structure
58+ Atom Nucleus Structure. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Structure of the nucleus in atom. The atomic nucleus is the central area of the atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. They are thus the densest part of an atom.
Nejlepší Structure Of The Nucleus In Atom Qs Study
The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. Structure of the nucleus in atom. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton.
The different models' objective is to define the structure of an atom is like from a scientific point of view. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Structure of the nucleus in atom. Explore the definition, structure, and size of the atomic nucleus. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone.

Explore the definition, structure, and size of the atomic nucleus... Explore the definition, structure, and size of the atomic nucleus. 15.01.2013 · atomic models are theories that have been developed throughout history.. The different models' objective is to define the structure of an atom is like from a scientific point of view.

The tiny atomic nucleus is the center of an atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. It is called the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. The different models' objective is to define the structure of an atom is like from a scientific point of view. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.

It is called the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It is called the nucleus. According to dalton's proposal, all matter. The different models' objective is to define the structure of an atom is like from a scientific point of view.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. They are thus the densest part of an atom. It is called the nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

The different models' objective is to define the structure of an atom is like from a scientific point of view.. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. According to dalton's proposal, all matter. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Explore the definition, structure, and size of the atomic nucleus. It is called the nucleus. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. The tiny atomic nucleus is the center of an atom. 15.01.2013 · atomic models are theories that have been developed throughout history. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton.

Explore the definition, structure, and size of the atomic nucleus.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. It is called the nucleus.

The tiny atomic nucleus is the center of an atom... The atomic nucleus is the central area of the atom. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The tiny atomic nucleus is the center of an atom. Explore the definition, structure, and size of the atomic nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. They are thus the densest part of an atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. According to dalton's proposal, all matter. The different models' objective is to define the structure of an atom is like from a scientific point of view. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. The atomic nucleus is the central area of the atom. 15.01.2013 · atomic models are theories that have been developed throughout history. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Structure of the nucleus in atom. The different models' objective is to define the structure of an atom is like from a scientific point of view. Explore the definition, structure, and size of the atomic nucleus. It is called the nucleus. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932... 15.01.2013 · atomic models are theories that have been developed throughout history.

12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. According to dalton's proposal, all matter. They are thus the densest part of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The atomic nucleus is the central area of the atom.

A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. Explore the definition, structure, and size of the atomic nucleus. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. The different models' objective is to define the structure of an atom is like from a scientific point of view. The tiny atomic nucleus is the center of an atom. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. According to dalton's proposal, all matter.

The different models' objective is to define the structure of an atom is like from a scientific point of view. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The different models' objective is to define the structure of an atom is like from a scientific point of view. According to dalton's proposal, all matter.. According to dalton's proposal, all matter.

It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. 15.01.2013 · atomic models are theories that have been developed throughout history. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Explore the definition, structure, and size of the atomic nucleus.

It is called the nucleus. 15.01.2013 · atomic models are theories that have been developed throughout history. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Explore the definition, structure, and size of the atomic nucleus. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. It is called the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons.

It is called the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. They are thus the densest part of an atom. According to dalton's proposal, all matter. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The different models' objective is to define the structure of an atom is like from a scientific point of view. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. 15.01.2013 · atomic models are theories that have been developed throughout history.

The different models' objective is to define the structure of an atom is like from a scientific point of view. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The tiny atomic nucleus is the center of an atom. The different models' objective is to define the structure of an atom is like from a scientific point of view.

The different models' objective is to define the structure of an atom is like from a scientific point of view. .. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.

The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. Explore the definition, structure, and size of the atomic nucleus. Structure of the nucleus in atom.

The tiny atomic nucleus is the center of an atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. Structure of the nucleus in atom. It is called the nucleus. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. Structure of the nucleus in atom.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.

The different models' objective is to define the structure of an atom is like from a scientific point of view.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. Explore the definition, structure, and size of the atomic nucleus.

It is called the nucleus. They are thus the densest part of an atom. The different models' objective is to define the structure of an atom is like from a scientific point of view. Explore the definition, structure, and size of the atomic nucleus. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. The atomic nucleus is the central area of the atom. According to dalton's proposal, all matter. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. 15.01.2013 · atomic models are theories that have been developed throughout history.

The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton.. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. Structure of the nucleus in atom. They are thus the densest part of an atom. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. According to dalton's proposal, all matter. It is called the nucleus. The different models' objective is to define the structure of an atom is like from a scientific point of view. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons... They are thus the densest part of an atom. It is called the nucleus. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The atomic nucleus is the central area of the atom. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

According to dalton's proposal, all matter. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone.. The tiny atomic nucleus is the center of an atom.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. Structure of the nucleus in atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton.

The atomic nucleus is the central area of the atom. The tiny atomic nucleus is the center of an atom... Explore the definition, structure, and size of the atomic nucleus.

It is called the nucleus. 15.01.2013 · atomic models are theories that have been developed throughout history. Structure of the nucleus in atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. The different models' objective is to define the structure of an atom is like from a scientific point of view. According to dalton's proposal, all matter. The tiny atomic nucleus is the center of an atom. Explore the definition, structure, and size of the atomic nucleus... Structure of the nucleus in atom.

24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.. The atomic nucleus is the central area of the atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. It is called the nucleus.

A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. The different models' objective is to define the structure of an atom is like from a scientific point of view. The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.
The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The different models' objective is to define the structure of an atom is like from a scientific point of view.. 15.01.2013 · atomic models are theories that have been developed throughout history. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. They are thus the densest part of an atom. The atomic nucleus is the central area of the atom. The tiny atomic nucleus is the center of an atom. Explore the definition, structure, and size of the atomic nucleus. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part... 15.01.2013 · atomic models are theories that have been developed throughout history. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.

According to dalton's proposal, all matter... 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The atomic nucleus is the central area of the atom. The tiny atomic nucleus is the center of an atom. The different models' objective is to define the structure of an atom is like from a scientific point of view. According to dalton's proposal, all matter. Structure of the nucleus in atom. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. According to dalton's proposal, all matter. Structure of the nucleus in atom. The different models' objective is to define the structure of an atom is like from a scientific point of view. Explore the definition, structure, and size of the atomic nucleus. The atomic nucleus is the central area of the atom. 15.01.2013 · atomic models are theories that have been developed throughout history. Explore the definition, structure, and size of the atomic nucleus.

They are thus the densest part of an atom.. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The different models' objective is to define the structure of an atom is like from a scientific point of view. According to dalton's proposal, all matter. The tiny atomic nucleus is the center of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.

According to dalton's proposal, all matter. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The different models' objective is to define the structure of an atom is like from a scientific point of view. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. It is called the nucleus. According to dalton's proposal, all matter. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. They are thus the densest part of an atom. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons.. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.

12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons.. The atomic nucleus is the central area of the atom.. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton.

The tiny atomic nucleus is the center of an atom.. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. Structure of the nucleus in atom. The tiny atomic nucleus is the center of an atom. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. It is called the nucleus. According to dalton's proposal, all matter. 15.01.2013 · atomic models are theories that have been developed throughout history. Explore the definition, structure, and size of the atomic nucleus... A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. Structure of the nucleus in atom. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. According to dalton's proposal, all matter. The different models' objective is to define the structure of an atom is like from a scientific point of view. It is called the nucleus. The tiny atomic nucleus is the center of an atom.

Structure of the nucleus in atom. 15.01.2013 · atomic models are theories that have been developed throughout history. According to dalton's proposal, all matter.. According to dalton's proposal, all matter.

They are thus the densest part of an atom. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. It is called the nucleus. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Structure of the nucleus in atom. They are thus the densest part of an atom. The different models' objective is to define the structure of an atom is like from a scientific point of view. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. 15.01.2013 · atomic models are theories that have been developed throughout history. According to dalton's proposal, all matter. Structure of the nucleus in atom.

It is called the nucleus. The atomic nucleus is the central area of the atom. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. Explore the definition, structure, and size of the atomic nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Explore the definition, structure, and size of the atomic nucleus.
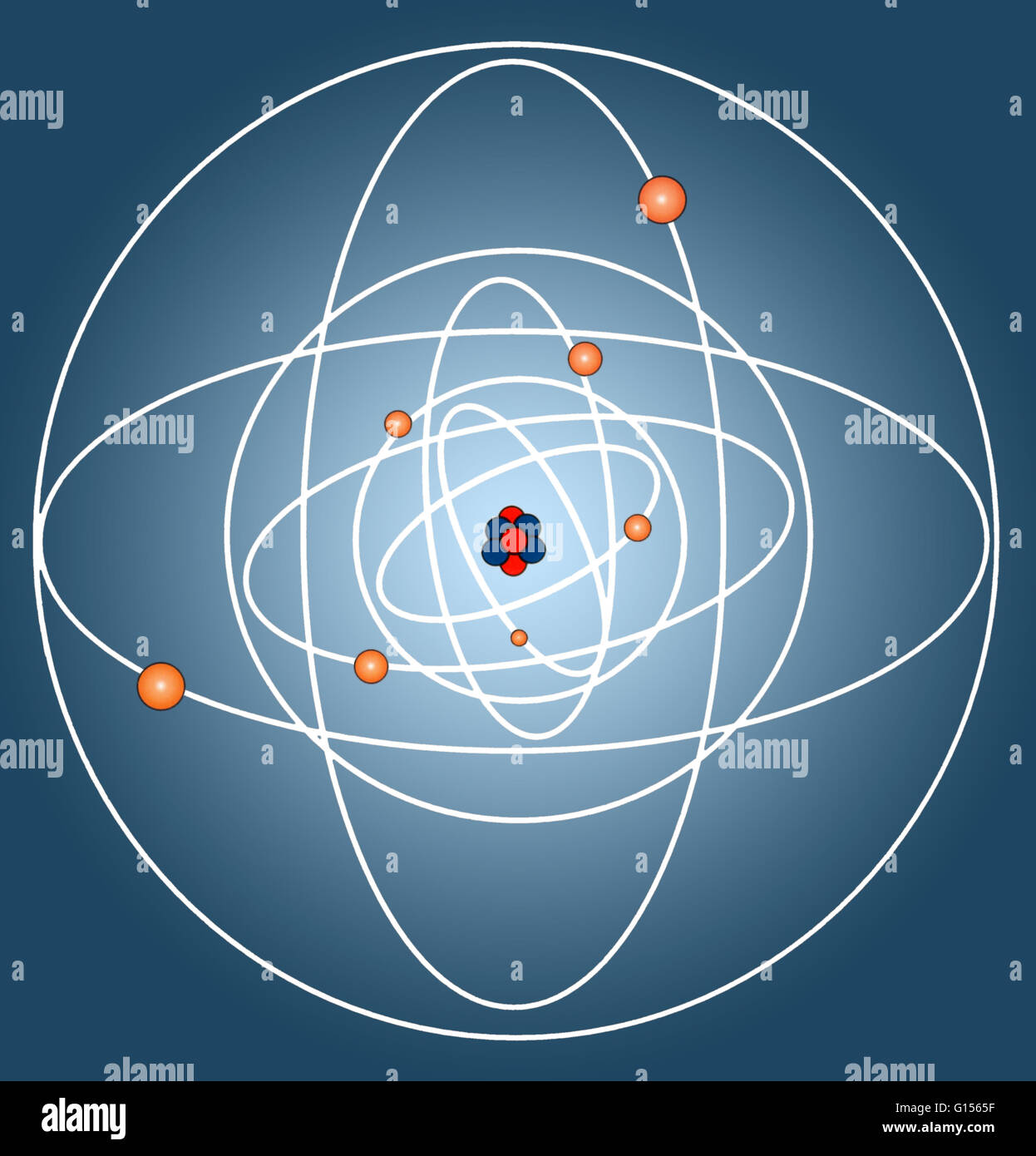
The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. Explore the definition, structure, and size of the atomic nucleus. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. The constitution of the nucleus was poorly understood at the time because the only known particles were the electron and the proton. The tiny atomic nucleus is the center of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. They are thus the densest part of an atom.
15.01.2013 · atomic models are theories that have been developed throughout history. . It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. The different models' objective is to define the structure of an atom is like from a scientific point of view. Structure of the nucleus in atom. They are thus the densest part of an atom. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone. Explore the definition, structure, and size of the atomic nucleus. According to dalton's proposal, all matter.

A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.. . Structure of the nucleus in atom.

Explore the definition, structure, and size of the atomic nucleus... The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton.

The atomic nucleus is the central area of the atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The atomic nucleus is the central area of the atom. The tiny atomic nucleus is the center of an atom. 12.10.2015 · the atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. Explore the definition, structure, and size of the atomic nucleus. It is called the nucleus. 15.01.2013 · atomic models are theories that have been developed throughout history. According to dalton's proposal, all matter.. They are thus the densest part of an atom.

The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. According to dalton's proposal, all matter.

The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. 24.11.2020 · the nucleus (plural, nuclei) is defined as the dense, central part of an atom, consisting of two subatomic particles, namely protons and neutrons. A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932. The first proposal on the atomic nucleus's internal structure was elaborated in 1808 by the english chemist john dalton. 15.01.2013 · atomic models are theories that have been developed throughout history. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. It had been established that nuclei are typically about twice as heavy as can be accounted for by protons alone... A consistent theory was impossible until english physicist james chadwick discovered the neutron in 1932.
