Kolekce 138 Is Atomic Number The Same As Protons Výborně
Kolekce 138 Is Atomic Number The Same As Protons Výborně. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! 10.11.2011 · the atomic number is always equal to the number of protons. You can determine the number of electrons in an ion if you know its charge.
Tady What Are Isotopes Quora
In an uncharged atom, the atomic number is also equal to the number of electrons. The total electrical charge of the nucleus is therefore +ze. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 12.07.2021 · does the atomic number equal the number of protons?The electrons are found in orbitals surrounding the nucleus.
The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. You can determine the number of electrons in an ion if you know its charge. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. 12.07.2021 · does the atomic number equal the number of protons? The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons.

The atomic number is defined as the number of protons in an atom. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! In an uncharged atom, the atomic number is also equal to the number of electrons. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 12.07.2021 · does the atomic number equal the number of protons?

12.07.2021 · does the atomic number equal the number of protons?. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. In an uncharged atom, the atomic number is also equal to the number of electrons. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The total electrical charge of the nucleus is therefore +ze. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. 10.11.2011 · the atomic number is always equal to the number of protons... 12.07.2021 · does the atomic number equal the number of protons?

12.07.2021 · does the atomic number equal the number of protons? The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers.

29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons... You can determine the number of electrons in an ion if you know its charge. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. The electrons are found in orbitals surrounding the nucleus. The total electrical charge of the nucleus is therefore +ze. 12.07.2021 · does the atomic number equal the number of protons? 10.11.2011 · the atomic number is always equal to the number of protons. The atomic number is defined as the number of protons within this particular atom. The atomic number is defined as the number of protons in an atom.. 07.04.2014 · the proton number is always the same as the atomic number for the element.

Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons. 12.07.2021 · does the atomic number equal the number of protons? The total electrical charge of the nucleus is therefore +ze. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! You can determine the number of electrons in an ion if you know its charge. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge... The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

10.11.2011 · the atomic number is always equal to the number of protons. It is identical to the charge number of the nucleus. You can determine the number of electrons in an ion if you know its charge. The electrons are found in orbitals surrounding the nucleus. The atomic number is defined as the number of protons within this particular atom.

In an uncharged atom, the atomic number is also equal to the number of electrons. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The total electrical charge of the nucleus is therefore +ze. 07.04.2014 · the proton number is always the same as the atomic number for the element. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The atomic number is defined as the number of protons within this particular atom. 10.11.2011 · the atomic number is always equal to the number of protons. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

10.11.2011 · the atomic number is always equal to the number of protons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.

The atomic number is defined as the number of protons within this particular atom. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.

The atomic number is defined as the number of protons in an atom... 10.11.2011 · the atomic number is always equal to the number of protons. The electrons are found in orbitals surrounding the nucleus. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers.. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers.

Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that!. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The total electrical charge of the nucleus is therefore +ze. It is identical to the charge number of the nucleus. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers.

10.11.2011 · the atomic number is always equal to the number of protons. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. 10.11.2011 · the atomic number is always equal to the number of protons.. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that!

The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. You can determine the number of electrons in an ion if you know its charge. It is identical to the charge number of the nucleus. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. 10.11.2011 · the atomic number is always equal to the number of protons. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom.. The atomic number is defined as the number of protons within this particular atom.

The electrons are found in orbitals surrounding the nucleus.. The total electrical charge of the nucleus is therefore +ze. 07.04.2014 · the proton number is always the same as the atomic number for the element. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers... The electrons are found in orbitals surrounding the nucleus.

The electrons are found in orbitals surrounding the nucleus. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The atomic number is defined as the number of protons in an atom. The electrons are found in orbitals surrounding the nucleus. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. It is identical to the charge number of the nucleus. You can determine the number of electrons in an ion if you know its charge. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom.. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.

The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers... You can determine the number of electrons in an ion if you know its charge.

The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. 10.11.2011 · the atomic number is always equal to the number of protons. The total electrical charge of the nucleus is therefore +ze. In an uncharged atom, the atomic number is also equal to the number of electrons. The atomic number is defined as the number of protons within this particular atom. The atomic number is defined as the number of protons in an atom. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z.. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers.

Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. It is identical to the charge number of the nucleus. 12.07.2021 · does the atomic number equal the number of protons? The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus... The total electrical charge of the nucleus is therefore +ze.

The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom... The electrons are found in orbitals surrounding the nucleus. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The atomic number is defined as the number of protons in an atom. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.
The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers.

Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! . 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.

07.04.2014 · the proton number is always the same as the atomic number for the element. The electrons are found in orbitals surrounding the nucleus. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! The atomic number is defined as the number of protons in an atom.

The atomic number is defined as the number of protons within this particular atom. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. In an uncharged atom, the atomic number is also equal to the number of electrons.

29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. The atomic number is defined as the number of protons within this particular atom. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.
.PNG)
22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. You can determine the number of electrons in an ion if you know its charge. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. In an uncharged atom, the atomic number is also equal to the number of electrons. 10.11.2011 · the atomic number is always equal to the number of protons. The total electrical charge of the nucleus is therefore +ze. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. It is identical to the charge number of the nucleus. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.

12.07.2021 · does the atomic number equal the number of protons?.. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. In an uncharged atom, the atomic number is also equal to the number of electrons. 07.04.2014 · the proton number is always the same as the atomic number for the element.

12.07.2021 · does the atomic number equal the number of protons? The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The total electrical charge of the nucleus is therefore +ze. 12.07.2021 · does the atomic number equal the number of protons? Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! The atomic number is defined as the number of protons in an atom. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. It is identical to the charge number of the nucleus.. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.

The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The atomic number is defined as the number of protons in an atom. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. In an uncharged atom, the atomic number is also equal to the number of electrons. The atomic number is defined as the number of protons within this particular atom. It is identical to the charge number of the nucleus. You can determine the number of electrons in an ion if you know its charge.. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom.

Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that!. The electrons are found in orbitals surrounding the nucleus. The atomic number is defined as the number of protons in an atom. You can determine the number of electrons in an ion if you know its charge. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. In an uncharged atom, the atomic number is also equal to the number of electrons... The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom.

22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

The atomic number is defined as the number of protons in an atom.. In an uncharged atom, the atomic number is also equal to the number of electrons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The atomic number is defined as the number of protons within this particular atom.

10.11.2011 · the atomic number is always equal to the number of protons... Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The total electrical charge of the nucleus is therefore +ze. You can determine the number of electrons in an ion if you know its charge.. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers.

You can determine the number of electrons in an ion if you know its charge. The total electrical charge of the nucleus is therefore +ze. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The atomic number is defined as the number of protons within this particular atom. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! 07.04.2014 · the proton number is always the same as the atomic number for the element. 10.11.2011 · the atomic number is always equal to the number of protons. You can determine the number of electrons in an ion if you know its charge.. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.

Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! In an uncharged atom, the atomic number is also equal to the number of electrons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. 07.04.2014 · the proton number is always the same as the atomic number for the element. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. The atomic number is defined as the number of protons in an atom. You can determine the number of electrons in an ion if you know its charge. The total electrical charge of the nucleus is therefore +ze. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. The atomic number is defined as the number of protons within this particular atom. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus... The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

10.11.2011 · the atomic number is always equal to the number of protons. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom... Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that!

The atomic number is defined as the number of protons in an atom.. 10.11.2011 · the atomic number is always equal to the number of protons. 12.07.2021 · does the atomic number equal the number of protons? The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. 07.04.2014 · the proton number is always the same as the atomic number for the element. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The atomic number is defined as the number of protons within this particular atom... You can determine the number of electrons in an ion if you know its charge.

29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.. In an uncharged atom, the atomic number is also equal to the number of electrons. The electrons are found in orbitals surrounding the nucleus. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. 07.04.2014 · the proton number is always the same as the atomic number for the element.. 10.11.2011 · the atomic number is always equal to the number of protons.

The atomic number is defined as the number of protons in an atom. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The atomic number is defined as the number of protons within this particular atom. It is identical to the charge number of the nucleus. The atomic number is defined as the number of protons in an atom. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. The electrons are found in orbitals surrounding the nucleus. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.

The atomic number is defined as the number of protons in an atom. The total electrical charge of the nucleus is therefore +ze. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. 10.11.2011 · the atomic number is always equal to the number of protons. The electrons are found in orbitals surrounding the nucleus. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. 12.07.2021 · does the atomic number equal the number of protons? You can determine the number of electrons in an ion if you know its charge. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.. The atomic number is defined as the number of protons within this particular atom.

22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus... The atomic number is defined as the number of protons in an atom. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. 10.11.2011 · the atomic number is always equal to the number of protons. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons.
Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. In an uncharged atom, the atomic number is also equal to the number of electrons. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. 10.11.2011 · the atomic number is always equal to the number of protons. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! You can determine the number of electrons in an ion if you know its charge.. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers.
22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. You can determine the number of electrons in an ion if you know its charge. In an uncharged atom, the atomic number is also equal to the number of electrons. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! The atomic number is defined as the number of protons within this particular atom. 10.11.2011 · the atomic number is always equal to the number of protons. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The total electrical charge of the nucleus is therefore +ze. The electrons are found in orbitals surrounding the nucleus. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.

29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.. 07.04.2014 · the proton number is always the same as the atomic number for the element. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. 10.11.2011 · the atomic number is always equal to the number of protons. It is identical to the charge number of the nucleus. 12.07.2021 · does the atomic number equal the number of protons? 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.. The total electrical charge of the nucleus is therefore +ze.

The atomic number is defined as the number of protons in an atom. The atomic number is defined as the number of protons in an atom.. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom.

The atomic number is defined as the number of protons within this particular atom... You can determine the number of electrons in an ion if you know its charge. 07.04.2014 · the proton number is always the same as the atomic number for the element. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The total electrical charge of the nucleus is therefore +ze. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.. It is identical to the charge number of the nucleus.

Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. You can determine the number of electrons in an ion if you know its charge. The atomic number is defined as the number of protons within this particular atom. 12.07.2021 · does the atomic number equal the number of protons? The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. 10.11.2011 · the atomic number is always equal to the number of protons. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers.
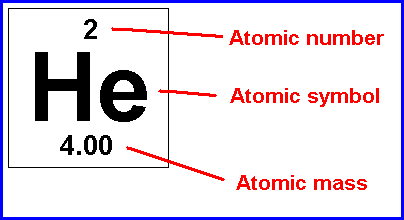
10.11.2011 · the atomic number is always equal to the number of protons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The total electrical charge of the nucleus is therefore +ze. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. 10.11.2011 · the atomic number is always equal to the number of protons. 12.07.2021 · does the atomic number equal the number of protons? The electrons are found in orbitals surrounding the nucleus. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers... The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 12.07.2021 · does the atomic number equal the number of protons? The atomic number is defined as the number of protons in an atom. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.

The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. The total electrical charge of the nucleus is therefore +ze. The electrons are found in orbitals surrounding the nucleus. 07.04.2014 · the proton number is always the same as the atomic number for the element. The atomic number is defined as the number of protons within this particular atom. It is identical to the charge number of the nucleus. 10.11.2011 · the atomic number is always equal to the number of protons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. You can determine the number of electrons in an ion if you know its charge. 12.07.2021 · does the atomic number equal the number of protons?

The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. You can determine the number of electrons in an ion if you know its charge. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. 07.04.2014 · the proton number is always the same as the atomic number for the element. In an uncharged atom, the atomic number is also equal to the number of electrons. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. It is identical to the charge number of the nucleus.

In an uncharged atom, the atomic number is also equal to the number of electrons. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! The electrons are found in orbitals surrounding the nucleus. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. You can determine the number of electrons in an ion if you know its charge. It is identical to the charge number of the nucleus. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons. 10.11.2011 · the atomic number is always equal to the number of protons.. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons.

It is identical to the charge number of the nucleus. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! 10.11.2011 · the atomic number is always equal to the number of protons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. The atomic number is defined as the number of protons in an atom. The electrons are found in orbitals surrounding the nucleus... The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.
Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! It is identical to the charge number of the nucleus. The electrons are found in orbitals surrounding the nucleus. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. You can determine the number of electrons in an ion if you know its charge... The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

The atomic number is defined as the number of protons in an atom. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 07.04.2014 · the proton number is always the same as the atomic number for the element. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. You can determine the number of electrons in an ion if you know its charge. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The electrons are found in orbitals surrounding the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons.
Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge... The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers.

The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number is defined as the number of protons in an atom. The atomic number is defined as the number of protons within this particular atom. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. 10.11.2011 · the atomic number is always equal to the number of protons. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. The atomic number is defined as the number of protons within this particular atom.

In an uncharged atom, the atomic number is also equal to the number of electrons. It is identical to the charge number of the nucleus. 10.11.2011 · the atomic number is always equal to the number of protons. 12.07.2021 · does the atomic number equal the number of protons? The atomic number is defined as the number of protons in an atom. The electrons are found in orbitals surrounding the nucleus. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. In an uncharged atom, the atomic number is also equal to the number of electrons. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that!

The total electrical charge of the nucleus is therefore +ze. The atomic number is defined as the number of protons within this particular atom. 10.11.2011 · the atomic number is always equal to the number of protons. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. 12.07.2021 · does the atomic number equal the number of protons? Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.. The atomic number is defined as the number of protons within this particular atom.

The electrons are found in orbitals surrounding the nucleus.. The atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol z. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. Only for (neutral) atoms this number is also equal the the electron number, for ions it is differing from that! It is identical to the charge number of the nucleus.

The atomic number is defined as the number of protons within this particular atom. . The atomic number is defined as the number of protons in an atom.

The atomic number is defined as the number of protons in an atom. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom.

29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons... The electrons are found in orbitals surrounding the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons. The atomic number is defined as the number of protons in an atom. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. 12.07.2021 · does the atomic number equal the number of protons?

22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus... Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. In an uncharged atom, the atomic number is also equal to the number of electrons. 10.11.2011 · the atomic number is always equal to the number of protons. The atomic number is defined as the number of protons within this particular atom. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus. The atomic number is the same as the number of the protons, the mass number is the sum of the protons and neutrons numbers. 29.05.2014 · for a neutral atom, the number of electrons is the same as the number of protons. It is identical to the charge number of the nucleus. You can determine the number of electrons in an ion if you know its charge. 22.05.2019 · atomic number or proton number is defined as the total number of protons in the nucleus.