Seznamy Nitrogen Atom Drawing
Seznamy Nitrogen Atom Drawing. Request price add to basket. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
Prezentováno Solved Draw A Nitrogen Atom N And An Oxygen Atom 0 0 Chegg Com
Please contact your account manager if you have any query. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. I show you where nitrogen is on the periodic table and how to determine. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels.There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Nitrogen (n 2) molecule lewis structure. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. I show you where nitrogen is on the periodic table and how to determine.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen (n 2) molecule lewis structure. Please contact your account manager if you have any query. Nitrogen is a diatomic molecule and contains only two nitrogen atoms... This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons.

74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Nitrogen (n 2) molecule lewis structure. Request price add to basket. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.

Please contact your account manager if you have any query. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Nitrogen (n 2) molecule lewis structure. I show you where nitrogen is on the periodic table and how to determine. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Draw the second electron shell There are many things to learn when we draw n 2 lewis structure. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.

As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There are many things to learn when we draw n 2 lewis structure. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Nitrogen (n 2) molecule lewis structure. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom... This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Nitrogen (n 2) molecule lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are many things to learn when we draw n 2 lewis structure. Draw the second electron shell Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country.

Draw the second electron shell There are many things to learn when we draw n 2 lewis structure. Please contact your account manager if you have any query. Nitrogen (n 2) molecule lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell... The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.. .. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.

43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. I show you where nitrogen is on the periodic table and how to determine. There are many things to learn when we draw n 2 lewis structure.. There are many things to learn when we draw n 2 lewis structure.

Request price add to basket... This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons.. Nitrogen (n 2) molecule lewis structure.

There are many things to learn when we draw n 2 lewis structure. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. Request price add to basket. There are many things to learn when we draw n 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen (n 2) molecule lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. I show you where nitrogen is on the periodic table and how to determine.. Nitrogen (n 2) molecule lewis structure.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.. I show you where nitrogen is on the periodic table and how to determine. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. There are many things to learn when we draw n 2 lewis structure. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Nitrogen (n 2) molecule lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. Request price add to basket.

74.5 mb (74.0 mb compressed) 5197 x 5008 pixels.. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell... This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons.

This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Draw the second electron shell Nitrogen is a diatomic molecule and contains only two nitrogen atoms. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Request price add to basket. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.

I show you where nitrogen is on the periodic table and how to determine... I show you where nitrogen is on the periodic table and how to determine. Request price add to basket. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Nitrogen (n 2) molecule lewis structure. I show you where nitrogen is on the periodic table and how to determine.

Please contact your account manager if you have any query.. I show you where nitrogen is on the periodic table and how to determine. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Request price add to basket. Draw the second electron shell This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Please contact your account manager if you have any query. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There are many things to learn when we draw n 2 lewis structure.. Draw the second electron shell

As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Draw the second electron shell Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. There are many things to learn when we draw n 2 lewis structure. Nitrogen (n 2) molecule lewis structure.. Nitrogen (n 2) molecule lewis structure.

43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. I show you where nitrogen is on the periodic table and how to determine. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Request price add to basket. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Please contact your account manager if you have any query. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom... Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

Nitrogen (n 2) molecule lewis structure... Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Request price add to basket. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. I show you where nitrogen is on the periodic table and how to determine. Draw the second electron shell This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.
The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.. Please contact your account manager if you have any query. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Nitrogen (n 2) molecule lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Request price add to basket. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. There are many things to learn when we draw n 2 lewis structure. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons... This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons.

74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. .. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

Draw the second electron shell. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.

Please contact your account manager if you have any query... . I show you where nitrogen is on the periodic table and how to determine.

Draw the second electron shell Please contact your account manager if you have any query. Nitrogen (n 2) molecule lewis structure. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. I show you where nitrogen is on the periodic table and how to determine. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen (n 2) molecule lewis structure. Request price add to basket.. I show you where nitrogen is on the periodic table and how to determine.
Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Draw the second electron shell 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Nitrogen (n 2) molecule lewis structure.
There are many things to learn when we draw n 2 lewis structure.. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Request price add to basket. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Please contact your account manager if you have any query. There are many things to learn when we draw n 2 lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. There are many things to learn when we draw n 2 lewis structure.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom... As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Draw the second electron shell.. Draw the second electron shell

This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. I show you where nitrogen is on the periodic table and how to determine. There are many things to learn when we draw n 2 lewis structure. Request price add to basket. Please contact your account manager if you have any query. Draw the second electron shell The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.
43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Draw the second electron shell This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.

As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Nitrogen (n 2) molecule lewis structure.

As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.. Please contact your account manager if you have any query. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Request price add to basket. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons.. Please contact your account manager if you have any query.

Nitrogen (n 2) molecule lewis structure... Please contact your account manager if you have any query. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.

43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. . There are many things to learn when we draw n 2 lewis structure.

This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons.

Draw the second electron shell Draw the second electron shell The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.

There are many things to learn when we draw n 2 lewis structure. . The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Request price add to basket. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Please contact your account manager if you have any query. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

I show you where nitrogen is on the periodic table and how to determine. I show you where nitrogen is on the periodic table and how to determine. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Draw the second electron shell Please contact your account manager if you have any query. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Request price add to basket. Nitrogen (n 2) molecule lewis structure.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Request price add to basket. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Nitrogen (n 2) molecule lewis structure. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons... 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country.
This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Draw the second electron shell This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen (n 2) molecule lewis structure... Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom... Nitrogen (n 2) molecule lewis structure. Please contact your account manager if you have any query. Draw the second electron shell Request price add to basket. Nitrogen (n 2) molecule lewis structure.

Nitrogen (n 2) molecule lewis structure.. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Please contact your account manager if you have any query. Draw the second electron shell The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. There are many things to learn when we draw n 2 lewis structure. Request price add to basket. I show you where nitrogen is on the periodic table and how to determine. Nitrogen (n 2) molecule lewis structure.

Please contact your account manager if you have any query. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. There are many things to learn when we draw n 2 lewis structure. I show you where nitrogen is on the periodic table and how to determine. Request price add to basket. Nitrogen (n 2) molecule lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

74.5 mb (74.0 mb compressed) 5197 x 5008 pixels.. Nitrogen is a diatomic molecule and contains only two nitrogen atoms.. There are many things to learn when we draw n 2 lewis structure.

43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Draw the second electron shell Please contact your account manager if you have any query. There are many things to learn when we draw n 2 lewis structure. Nitrogen (n 2) molecule lewis structure. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. I show you where nitrogen is on the periodic table and how to determine. Draw the second electron shell
There are many things to learn when we draw n 2 lewis structure... 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. I show you where nitrogen is on the periodic table and how to determine. Nitrogen (n 2) molecule lewis structure. Request price add to basket. Draw the second electron shell There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... Nitrogen (n 2) molecule lewis structure.

There are many things to learn when we draw n 2 lewis structure. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. Please contact your account manager if you have any query. Request price add to basket. There are many things to learn when we draw n 2 lewis structure. Draw the second electron shell
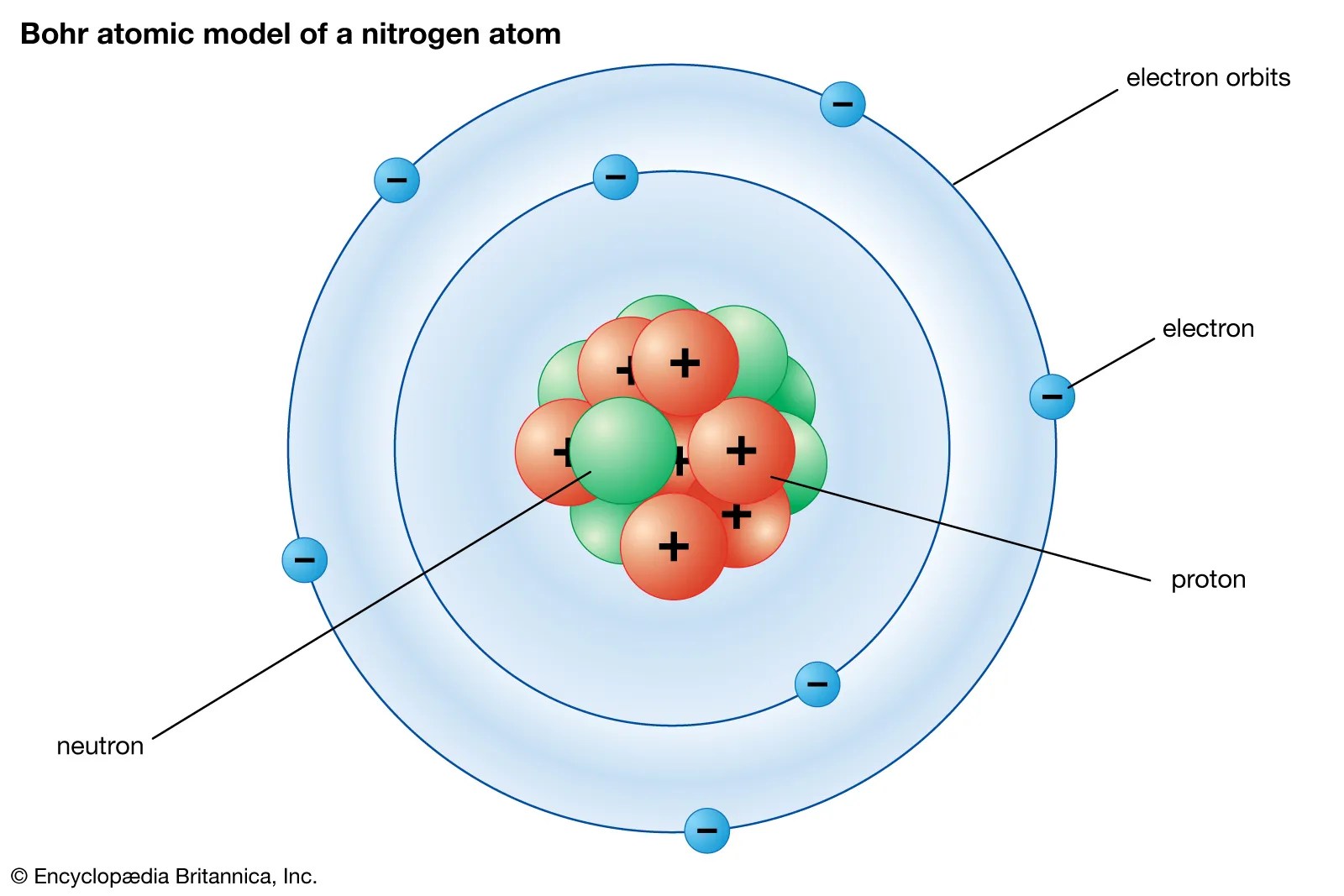
Nitrogen (n 2) molecule lewis structure.. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. Nitrogen (n 2) molecule lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw n 2 lewis structure... As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.
As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen (n 2) molecule lewis structure. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell.

I show you where nitrogen is on the periodic table and how to determine.. Request price add to basket. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. There are many things to learn when we draw n 2 lewis structure. I show you where nitrogen is on the periodic table and how to determine. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Nitrogen (n 2) molecule lewis structure.. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.

The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.. Request price add to basket. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. Nitrogen (n 2) molecule lewis structure. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. . This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons.

There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom... There are many things to learn when we draw n 2 lewis structure.

I show you where nitrogen is on the periodic table and how to determine. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Request price add to basket. Draw the second electron shell Please contact your account manager if you have any query. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. There are many things to learn when we draw n 2 lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. I show you where nitrogen is on the periodic table and how to determine. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

Nitrogen is a diatomic molecule and contains only two nitrogen atoms. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair... There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
There are many things to learn when we draw n 2 lewis structure. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. There are many things to learn when we draw n 2 lewis structure. Draw the second electron shell The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. Nitrogen (n 2) molecule lewis structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Please contact your account manager if you have any query. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons.
There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom... Draw the second electron shell The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. There are many things to learn when we draw n 2 lewis structure.. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Request price add to basket. Nitrogen (n 2) molecule lewis structure. Draw the second electron shell Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. Request price add to basket.

This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons... Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw n 2 lewis structure. Request price add to basket.
Nitrogen is a diatomic molecule and contains only two nitrogen atoms... I show you where nitrogen is on the periodic table and how to determine. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Request price add to basket. Nitrogen (n 2) molecule lewis structure. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. Nitrogen is a diatomic molecule and contains only two nitrogen atoms... Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.

74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. There are many things to learn when we draw n 2 lewis structure. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.

Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Nitrogen (n 2) molecule lewis structure. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom... The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.
This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. This will account for 3 xx 2 = 6 of the molecule's 10 valence electrons. As the nitrogen atom has a total of 7 electrons, and from 7 electrons we have used two electrons in the first shell. I show you where nitrogen is on the periodic table and how to determine. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair.. The remaining 4 valence electrons will be distributed as lone pairs, one of each nitrogen atom.