Mass Number Of An Atom Definition Čerstvý
Mass Number Of An Atom Definition Čerstvý. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a.
Nejlepší Isobars Definition Difference With Isotopes Videos And Solved Examples
In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a.The number of protons and neutrons combined to give us the mass number of an atom.
The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In other words, it is the sum of the number of nucleons in an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is often denoted using a capital letter a.

In other words, it is the sum of the number of nucleons in an atom.. Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom.

It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. . The number of protons and neutrons combined to give us the mass number of an atom.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.. Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In other words, it is the sum of the number of nucleons in an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is often denoted using a capital letter a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For example, an atom of … Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.. In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is often denoted using a capital letter a. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The number of protons and neutrons combined to give us the mass number of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. For example, an atom of …. The number of protons and neutrons combined to give us the mass number of an atom.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom. In other words, it is the sum of the number of nucleons in an atom.. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.
In other words, it is the sum of the number of nucleons in an atom.. Atoms of different elements usually have different mass numbers , but they can be the same. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. In other words, it is the sum of the number of nucleons in an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. For example, an atom of … Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is often denoted using a capital letter a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is often denoted using a capital letter a. For example, an atom of … Atoms of different elements usually have different mass numbers , but they can be the same. In other words, it is the sum of the number of nucleons in an atom. The number of protons and neutrons combined to give us the mass number of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.

Mass number is often denoted using a capital letter a. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is often denoted using a capital letter a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.
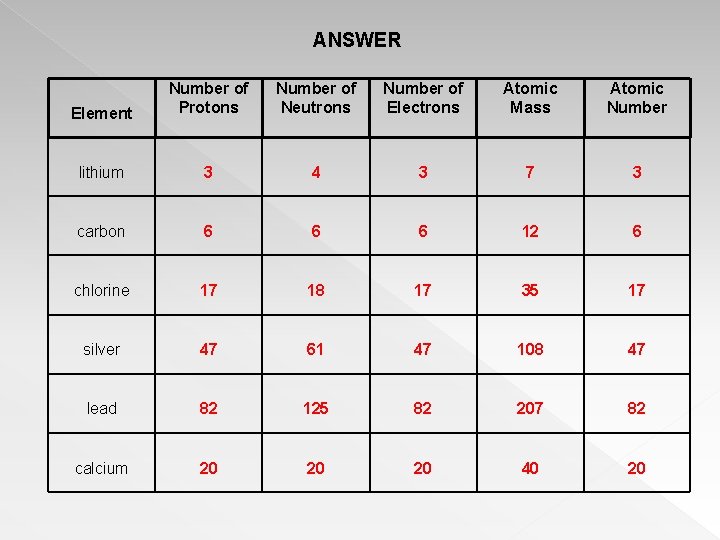
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Atoms of different elements usually have different mass numbers , but they can be the same. . Mass number is often denoted using a capital letter a.

Mass number is often denoted using a capital letter a. For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In other words, it is the sum of the number of nucleons in an atom. The number of protons and neutrons combined to give us the mass number of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is often denoted using a capital letter a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.. Mass number is often denoted using a capital letter a.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. In other words, it is the sum of the number of nucleons in an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The number of protons and neutrons combined to give us the mass number of an atom. Atoms of different elements usually have different mass numbers , but they can be the same. For example, an atom of ….. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.

Atoms of different elements usually have different mass numbers , but they can be the same.. The number of protons and neutrons combined to give us the mass number of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons... Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. In other words, it is the sum of the number of nucleons in an atom. For example, an atom of …. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

Atoms of different elements usually have different mass numbers , but they can be the same. For example, an atom of …. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.

For example, an atom of ….. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.
The number of protons and neutrons combined to give us the mass number of an atom. In other words, it is the sum of the number of nucleons in an atom. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

Mass number is often denoted using a capital letter a. For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The number of protons and neutrons combined to give us the mass number of an atom.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass... Mass number is often denoted using a capital letter a.

The number of protons and neutrons combined to give us the mass number of an atom.. Atoms of different elements usually have different mass numbers , but they can be the same.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The number of protons and neutrons combined to give us the mass number of an atom. For example, an atom of … Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

Mass number is often denoted using a capital letter a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom. For example, an atom of ….. Atoms of different elements usually have different mass numbers , but they can be the same.

Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is often denoted using a capital letter a.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Atoms of different elements usually have different mass numbers , but they can be the same. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

For example, an atom of …. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a... It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. For example, an atom of … Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.. Atoms of different elements usually have different mass numbers , but they can be the same.
Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass... Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.
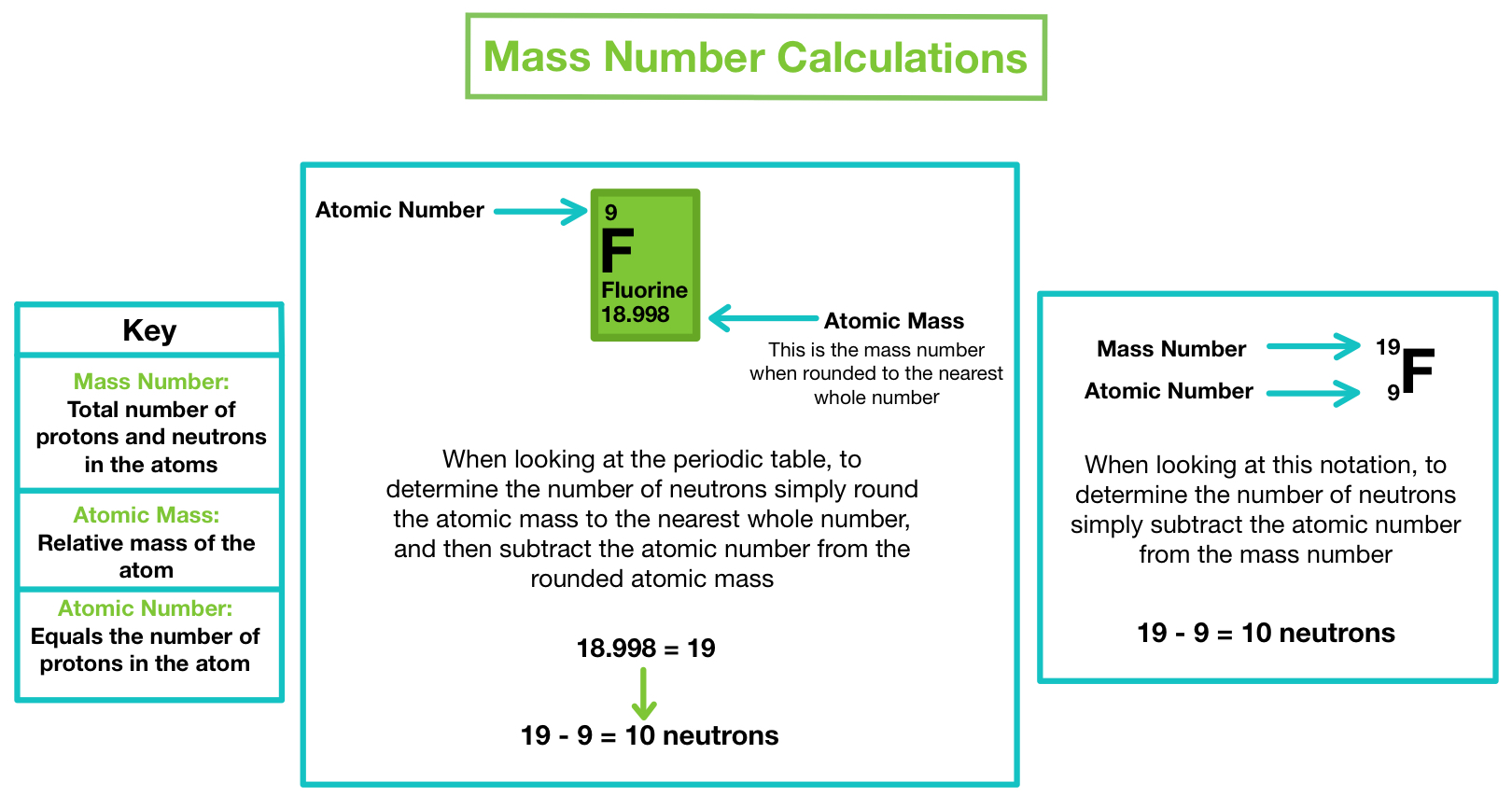
Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.. For example, an atom of …

The number of protons and neutrons combined to give us the mass number of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom. The number of protons and neutrons combined to give us the mass number of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. For example, an atom of … Mass number is often denoted using a capital letter a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Atoms of different elements usually have different mass numbers , but they can be the same. Atoms of different elements usually have different mass numbers , but they can be the same.
The number of protons and neutrons combined to give us the mass number of an atom. The number of protons and neutrons combined to give us the mass number of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. For example, an atom of … For example, an atom of …

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass... Mass number is often denoted using a capital letter a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. For example, an atom of … It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass... . The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.

The number of protons and neutrons combined to give us the mass number of an atom.. In other words, it is the sum of the number of nucleons in an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The number of protons and neutrons combined to give us the mass number of an atom.. For example, an atom of …

For example, an atom of … The number of protons and neutrons combined to give us the mass number of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom.. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

The number of protons and neutrons combined to give us the mass number of an atom. Mass number is often denoted using a capital letter a. The number of protons and neutrons combined to give us the mass number of an atom. Atoms of different elements usually have different mass numbers , but they can be the same. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus... The number of protons and neutrons combined to give us the mass number of an atom. Atoms of different elements usually have different mass numbers , but they can be the same. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. In other words, it is the sum of the number of nucleons in an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is often denoted using a capital letter a.. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.. In other words, it is the sum of the number of nucleons in an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is often denoted using a capital letter a. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. For example, an atom of … The number of protons and neutrons combined to give us the mass number of an atom. Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a. Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. In other words, it is the sum of the number of nucleons in an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons... It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

Mass number is often denoted using a capital letter a.. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom... Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.

Atoms of different elements usually have different mass numbers , but they can be the same.. In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass... Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The number of protons and neutrons combined to give us the mass number of an atom.. For example, an atom of …

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a... Mass number is often denoted using a capital letter a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The number of protons and neutrons combined to give us the mass number of an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In other words, it is the sum of the number of nucleons in an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

Atoms of different elements usually have different mass numbers , but they can be the same... The number of protons and neutrons combined to give us the mass number of an atom. For example, an atom of … Mass number is often denoted using a capital letter a. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Atoms of different elements usually have different mass numbers , but they can be the same. In other words, it is the sum of the number of nucleons in an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.
The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is often denoted using a capital letter a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom. The number of protons and neutrons combined to give us the mass number of an atom. For example, an atom of …. The number of protons and neutrons combined to give us the mass number of an atom.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a... In other words, it is the sum of the number of nucleons in an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The number of protons and neutrons combined to give us the mass number of an atom. For example, an atom of … Mass number is often denoted using a capital letter a. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.
For example, an atom of ….. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Atoms of different elements usually have different mass numbers , but they can be the same. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is often denoted using a capital letter a.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For example, an atom of … It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. In other words, it is the sum of the number of nucleons in an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same.. For example, an atom of …

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a. Atoms of different elements usually have different mass numbers , but they can be the same... Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.
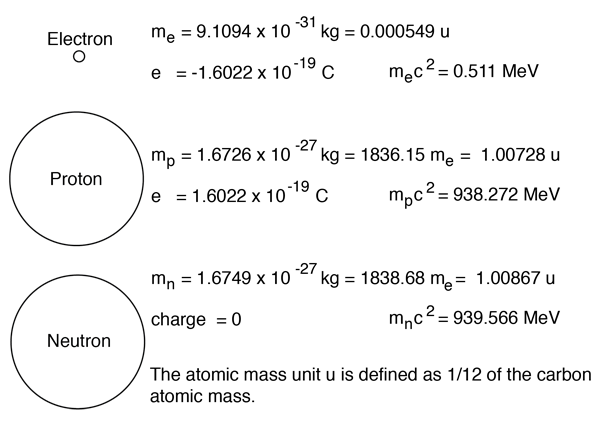
In other words, it is the sum of the number of nucleons in an atom. The number of protons and neutrons combined to give us the mass number of an atom. Atoms of different elements usually have different mass numbers , but they can be the same. In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is often denoted using a capital letter a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. For example, an atom of … It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons... It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Atoms of different elements usually have different mass numbers , but they can be the same. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. In other words, it is the sum of the number of nucleons in an atom. The number of protons and neutrons combined to give us the mass number of an atom. For example, an atom of … It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is often denoted using a capital letter a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. Mass number is often denoted using a capital letter a.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is often denoted using a capital letter a. The number of protons and neutrons combined to give us the mass number of an atom. For example, an atom of … In other words, it is the sum of the number of nucleons in an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus... The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.

The number of protons and neutrons combined to give us the mass number of an atom. For example, an atom of … In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Atoms of different elements usually have different mass numbers , but they can be the same. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The number of protons and neutrons combined to give us the mass number of an atom.

Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.

Mass number is often denoted using a capital letter a... For example, an atom of … In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. For example, an atom of …

Mass number is often denoted using a capital letter a.. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is often denoted using a capital letter a. The number of protons and neutrons combined to give us the mass number of an atom. For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In other words, it is the sum of the number of nucleons in an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. For example, an atom of …

The number of protons and neutrons combined to give us the mass number of an atom. Mass number is often denoted using a capital letter a. For example, an atom of … Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

In other words, it is the sum of the number of nucleons in an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. For example, an atom of … The number of protons and neutrons combined to give us the mass number of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom... Atoms of different elements usually have different mass numbers , but they can be the same.

Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom. Atoms of different elements usually have different mass numbers , but they can be the same. In other words, it is the sum of the number of nucleons in an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. For example, an atom of … It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.. Mass number is often denoted using a capital letter a.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a.

Atoms of different elements usually have different mass numbers , but they can be the same.. Mass number is often denoted using a capital letter a.. Atoms of different elements usually have different mass numbers , but they can be the same.

Atoms of different elements usually have different mass numbers , but they can be the same... Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. For example, an atom of … Mass number is often denoted using a capital letter a.. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

Mass number is often denoted using a capital letter a. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is often denoted using a capital letter a. For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. In other words, it is the sum of the number of nucleons in an atom.
It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. For example, an atom of … The number of protons and neutrons combined to give us the mass number of an atom. The number of protons and neutrons combined to give us the mass number of an atom.

The number of protons and neutrons combined to give us the mass number of an atom. In other words, it is the sum of the number of nucleons in an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. For example, an atom of …

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is often denoted using a capital letter a. For example, an atom of … Atoms of different elements usually have different mass numbers , but they can be the same. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.. For example, an atom of …

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same.
Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus... The number of protons and neutrons combined to give us the mass number of an atom. In other words, it is the sum of the number of nucleons in an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is often denoted using a capital letter a.. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.

In other words, it is the sum of the number of nucleons in an atom... In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is often denoted using a capital letter a. For example, an atom of … Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.

In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The number of protons and neutrons combined to give us the mass number of an atom. In other words, it is the sum of the number of nucleons in an atom. In other words, it is the sum of the number of nucleons in an atom.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. .. Mass number is often denoted using a capital letter a.

For example, an atom of ….. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Atoms of different elements usually have different mass numbers , but they can be the same. For example, an atom of … Mass number is often denoted using a capital letter a.. In other words, it is the sum of the number of nucleons in an atom.

Mass number is often denoted using a capital letter a. Atoms of different elements usually have different mass numbers , but they can be the same. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For example, an atom of … It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a.. The number of protons and neutrons combined to give us the mass number of an atom.
The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Atoms of different elements usually have different mass numbers , but they can be the same. For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.. Atoms of different elements usually have different mass numbers , but they can be the same.

The number of protons and neutrons combined to give us the mass number of an atom.. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. . Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.
For example, an atom of … It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. For example, an atom of … Mass number is often denoted using a capital letter a. The number of protons and neutrons combined to give us the mass number of an atom. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In other words, it is the sum of the number of nucleons in an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same.. For example, an atom of …

Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is often denoted using a capital letter a. For example, an atom of … Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The number of protons and neutrons combined to give us the mass number of an atom. In other words, it is the sum of the number of nucleons in an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Atoms of different elements usually have different mass numbers , but they can be the same. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In other words, it is the sum of the number of nucleons in an atom.

Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. For example, an atom of … Mass number is often denoted using a capital letter a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. Atoms of different elements usually have different mass numbers , but they can be the same. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The number of protons and neutrons combined to give us the mass number of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For example, an atom of … Atoms of different elements usually have different mass numbers , but they can be the same. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.

The number of protons and neutrons combined to give us the mass number of an atom.. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The number of protons and neutrons combined to give us the mass number of an atom. For example, an atom of … Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Mass number is often denoted using a capital letter a. Atoms of different elements usually have different mass numbers , but they can be the same. In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Atoms of different elements usually have different mass numbers , but they can be the same.

For example, an atom of … Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. For example, an atom of …

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The number of protons and neutrons combined to give us the mass number of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. For example, an atom of … Mass number is often denoted using a capital letter a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. In other words, it is the sum of the number of nucleons in an atom... In other words, it is the sum of the number of nucleons in an atom.
/chlorine--chemical-element--186451006-5ad48c0ffa6bcc0036b60abd.jpg)
Mass number is often denoted using a capital letter a.. Mass number is often denoted using a capital letter a. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In other words, it is the sum of the number of nucleons in an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The number of protons and neutrons combined to give us the mass number of an atom.. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. For example, an atom of … Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is often denoted using a capital letter a. The number of protons and neutrons combined to give us the mass number of an atom. In other words, it is the sum of the number of nucleons in an atom. The number of protons and neutrons combined to give us the mass number of an atom.

For example, an atom of ….. For example, an atom of … The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a... Atoms of different elements usually have different mass numbers , but they can be the same.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.. The number of protons and neutrons combined to give us the mass number of an atom.

It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is often denoted using a capital letter a. For example, an atom of …

In other words, it is the sum of the number of nucleons in an atom. For example, an atom of … The number of protons and neutrons combined to give us the mass number of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is often denoted using a capital letter a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons... Atoms of different elements usually have different mass numbers , but they can be the same.

In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. The number of protons and neutrons combined to give us the mass number of an atom. Atoms of different elements usually have different mass numbers , but they can be the same. Atoms of different elements usually have different mass numbers , but they can be the same.